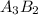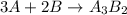Chemistry, 30.06.2019 14:30 sashajayne8260
You are balancing an ionic compound. let's call it: ab. ion a has a charge of +2 and ion b has a charge of -3. therefore, you determine that the ionic compound (ab) will have a ratio of 3 as for every 2 bs. how would you modify ab in order to show that they have this 3-to-2 ratio? question 2 options: 3a2b a3b2 a3b2

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1
You know the right answer?
You are balancing an ionic compound. let's call it: ab. ion a has a charge of +2 and ion b has a c...
Questions



Biology, 16.07.2019 07:00








Computers and Technology, 16.07.2019 07:00

History, 16.07.2019 07:00



Mathematics, 16.07.2019 07:00

Biology, 16.07.2019 07:00


English, 16.07.2019 07:00