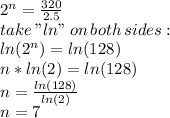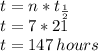Chemistry, 09.01.2020 03:31 mikaelalcool1
Me i need to turn this in really !
1. a chemical equation is shown below. hgs + o2 → hgo + so2 what are the coefficients that should be added to balance this equation? use complete sentences to explain your answer. explain how this chemical reaction demonstrates the conservation of mass.
2. part 1: name the type of chemical reaction that occurs when aluminum (al) reacts with copper nitrate (cu(no3)2).
part 2: explain why aluminum does not react with potassium nitrate (kno3) although it reacts with copper nitrate.
3.
the table shows the amount of radioactive element remaining in a sample over a period of time.
radioactive decay rate
amount of radioactive sample
(grams) time
(hours)
186.0 0
147.0 7
117.0 14
93.0 21
23.3 63
part 1: what is the half-life of the element? explain how you determined this.
part 2: how long would it take 320 g of the sample to decay to 2.5 grams? show your work or explain your answer.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1


You know the right answer?
Me i need to turn this in really !
1. a chemical equation is shown below. hgs + o2 → hg...
1. a chemical equation is shown below. hgs + o2 → hg...
Questions




Computers and Technology, 19.02.2020 23:34




History, 19.02.2020 23:34

Mathematics, 19.02.2020 23:34

History, 19.02.2020 23:34


World Languages, 19.02.2020 23:34

Mathematics, 19.02.2020 23:34

Biology, 19.02.2020 23:34



English, 19.02.2020 23:34

Social Studies, 19.02.2020 23:34


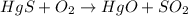
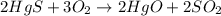
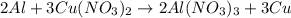
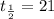 hours.
hours. --- (A)
--- (A)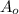 = initial mass of the sample = 320 g
= initial mass of the sample = 320 g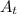 = Mass left after decaying (or after t amount of time) = 2.5 g
= Mass left after decaying (or after t amount of time) = 2.5 g