
Chemistry, 30.06.2019 18:30 dthompson365
For the following hypothetical reaction: 3a+> 12c, how many moles of c can you produce with 2 moles of a and excess b? (enter just the number; not the number and units)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

You know the right answer?
For the following hypothetical reaction: 3a+> 12c, how many moles of c can you produce with 2...
Questions

English, 28.06.2019 02:00



Mathematics, 28.06.2019 02:00

English, 28.06.2019 02:00

Mathematics, 28.06.2019 02:00

Computers and Technology, 28.06.2019 02:00


Arts, 28.06.2019 02:00

Mathematics, 28.06.2019 02:00




English, 28.06.2019 02:00

History, 28.06.2019 02:00




Physics, 28.06.2019 02:00

Mathematics, 28.06.2019 02:00

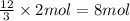 of compound C.
of compound C.

