

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2

Chemistry, 23.06.2019 07:00
Determine the length of the object shown. 97.8 mm 97.80 mm 97 mm 98 mm
Answers: 1
You know the right answer?
Asolution of ammonia and water contains 3.80•10^25 molecules of water and 5.50•10^24 molecules of am...
Questions

Mathematics, 27.01.2021 18:20

Mathematics, 27.01.2021 18:20

Mathematics, 27.01.2021 18:20




Mathematics, 27.01.2021 18:20



Mathematics, 27.01.2021 18:20



Mathematics, 27.01.2021 18:20

Mathematics, 27.01.2021 18:20

Mathematics, 27.01.2021 18:20

Mathematics, 27.01.2021 18:20

English, 27.01.2021 18:20

Mathematics, 27.01.2021 18:20


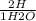 = 7.60×10²⁵atoms of H
= 7.60×10²⁵atoms of H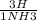 = 1.65×10²⁵atoms of H
= 1.65×10²⁵atoms of H

