
Chemistry, 01.07.2019 00:00 kianofou853
Kw, the equilibrium constant for the ionization of water by the equation below, is 1.0 x 10-14. what does that mean when we are considering pure water?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1
You know the right answer?
Kw, the equilibrium constant for the ionization of water by the equation below, is 1.0 x 10-14. what...
Questions





English, 22.12.2020 01:00


Advanced Placement (AP), 22.12.2020 01:00

Mathematics, 22.12.2020 01:00

Mathematics, 22.12.2020 01:00

Mathematics, 22.12.2020 01:00




Mathematics, 22.12.2020 01:00

Engineering, 22.12.2020 01:00




English, 22.12.2020 01:00

![[H^+]\&[OH^-]](/tpl/images/0036/7175/bc884.png) are equal in pure water.
are equal in pure water.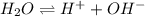
![K_{eq}=\frac{[H^+][OH^-]}{[H_2O]}](/tpl/images/0036/7175/59636.png)
![K_w=K_{eq}[H_2O]=[H^+][OH^-]=1.0\times 10^{-14}](/tpl/images/0036/7175/f0cd4.png)
![pH=7=-\log[H^+]](/tpl/images/0036/7175/ae186.png)
![[H^+]=1\times 10^{-7}mol/L](/tpl/images/0036/7175/f6d22.png)
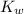 of pure water is given
of pure water is given![K_w=[H^+][OH^-]=1\times 10^{-7}\times[OH^-]= 1\times 10^{-14}](/tpl/images/0036/7175/db7b7.png)
![[OH^-]=1\times 10^{-7}mol/L](/tpl/images/0036/7175/b6b71.png)
![[H^+]\&[OH^-]](/tpl/images/0036/7175/72025.png) are equal in pure water
are equal in pure water

