

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:20
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1
You know the right answer?
What is the numerical value of kc for the following reaction if the equilibrium mixture contains 0.5...
Questions

Mathematics, 07.01.2020 06:31


History, 07.01.2020 06:31

History, 07.01.2020 06:31





Mathematics, 07.01.2020 06:31

Social Studies, 07.01.2020 06:31




Biology, 07.01.2020 06:31



Mathematics, 07.01.2020 06:31



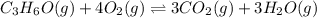
![Kc=\frac{[CO_2]^3[H_2O]^3}{[C_3H_6O][O_2]^4}](/tpl/images/0036/7459/52f34.png)
![Kc=\frac{[1.8]^3[2.0]^3}{[0.51][0.30]^4}](/tpl/images/0036/7459/c5aa6.png)


