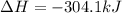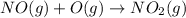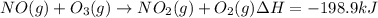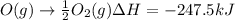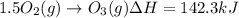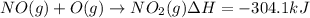Chemistry, 01.07.2019 00:30 jerseygirl1783
Calculate the enthalpy change for the reaction no(g) + o(g) → no2(g) from the following data: no(g) + o3(g) → no2(g) + o2(g) δh = –198.9 kj o3(g) → 1.5o2(g) δh = –142.3 kj o2(g) → 2o(g) δh = 495.0 kj

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3


Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
Calculate the enthalpy change for the reaction no(g) + o(g) → no2(g) from the following data: no(g)...
Questions


Social Studies, 09.10.2019 09:10

English, 09.10.2019 09:10

Chemistry, 09.10.2019 09:10

Business, 09.10.2019 09:10


Biology, 09.10.2019 09:10

History, 09.10.2019 09:10

Biology, 09.10.2019 09:10



Biology, 09.10.2019 09:10


History, 09.10.2019 09:10

Spanish, 09.10.2019 09:10


Mathematics, 09.10.2019 09:10


Biology, 09.10.2019 09:10

Mathematics, 09.10.2019 09:10