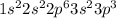Chemistry, 01.07.2019 00:30 baeethtsadia
(5 points) 2. a neutral atom of phosphorus has 15 electrons. explain why the electron configuration below is not the correct configuration for a neutral atom of phosphorus in its ground state. 1s2 2s2 2p6 3s2 3p2 4s1 (5 points) 3. find rubidium, magnesium, and aluminum on the periodic table. fill in the table below based on the locations of these metals on the periodic table. be thorough in filling in the far right column! element symbol group number number of valence electrons general reactivity of the metal with an explanation for this reactivity based on the number of valence electrons rubidium magnesium aluminum

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:40
Asingle atom of an element has 21 neutrons, 20 electrons, and 20 protons. which element is it? ok o z
Answers: 1

Chemistry, 22.06.2019 00:10
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1


Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1
You know the right answer?
(5 points) 2. a neutral atom of phosphorus has 15 electrons. explain why the electron configuration...
Questions

Medicine, 01.09.2020 05:01

Arts, 01.09.2020 05:01








History, 01.09.2020 05:01

Mathematics, 01.09.2020 05:01

Mathematics, 01.09.2020 05:01

Mathematics, 01.09.2020 05:01

Mathematics, 01.09.2020 05:01

Mathematics, 01.09.2020 05:01

Mathematics, 01.09.2020 05:01

Mathematics, 01.09.2020 05:01

Mathematics, 01.09.2020 05:01

Mathematics, 01.09.2020 05:01

Mathematics, 01.09.2020 05:01