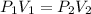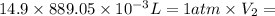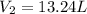Chemistry, 01.07.2019 03:30 artistictype4671
Agas storage cylinder in an ordinary chemical laboratory measures 6.6cm wide and 26.cm high. this is the label on it. n2 gas pressure: 14.9atm if the cylinder is opened and the gas allowed to escape into a large empty plastic bag, what will be the final volume of nitrogen gas, including what's collected in the plastic bag and what's left over in the cylinder? write your answer in liters. round your answer to 2 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2


Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
You know the right answer?
Agas storage cylinder in an ordinary chemical laboratory measures 6.6cm wide and 26.cm high. this is...
Questions

Mathematics, 20.06.2021 19:40




English, 20.06.2021 19:50


Mathematics, 20.06.2021 19:50

Business, 20.06.2021 19:50

Computers and Technology, 20.06.2021 19:50


English, 20.06.2021 19:50



Mathematics, 20.06.2021 19:50

Business, 20.06.2021 19:50



Mathematics, 20.06.2021 19:50

Social Studies, 20.06.2021 19:50

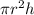
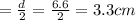
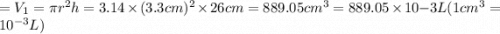
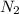 gas in a cylinder =
gas in a cylinder =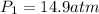
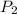 will become equal to atmospheric pressure that 1 atm .
will become equal to atmospheric pressure that 1 atm .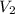 will be given by the expression of Boyle's law
will be given by the expression of Boyle's law