
Chemistry, 01.07.2019 08:30 andrea1704
The formation of cscl from cs(s) and cl2 (g) involves the following steps: cs(s) -> cs(g) 1/2cl2(g) -> cl(g) cs(g) -> cs + (g) + e – cl(g) + e - -> cl - (g) cs+(g) + cl - (g) -> cscl(s) which of these steps absorb energy and which release energy?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:50
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2
You know the right answer?
The formation of cscl from cs(s) and cl2 (g) involves the following steps: cs(s) -> cs(g) 1/2...
Questions

Social Studies, 04.03.2021 06:50

Mathematics, 04.03.2021 06:50

Mathematics, 04.03.2021 06:50

English, 04.03.2021 06:50


English, 04.03.2021 06:50


Mathematics, 04.03.2021 06:50

Social Studies, 04.03.2021 06:50

Social Studies, 04.03.2021 06:50


Mathematics, 04.03.2021 06:50

Mathematics, 04.03.2021 06:50

Mathematics, 04.03.2021 06:50


Mathematics, 04.03.2021 06:50


Mathematics, 04.03.2021 06:50


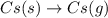
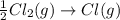
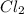 gas in order to break bond between two Cl atoms to form isolated(alone) single chlorine atom.So, the energy will be absorbed by the chlorine gas molecule in this reaction.
gas in order to break bond between two Cl atoms to form isolated(alone) single chlorine atom.So, the energy will be absorbed by the chlorine gas molecule in this reaction.
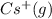 cation. In order to remove an electron from the outer most shell of Cs atom energy will be required by Cs atom, So, energy will be absorbed in this reaction.
cation. In order to remove an electron from the outer most shell of Cs atom energy will be required by Cs atom, So, energy will be absorbed in this reaction.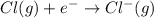
 anion from Cl atom in gaseous state. Chlorine atom need one electron to attain noble gas configuration. So, when an electron is added to the outer most shell of chlorine it attains stability of fully filled outermost shell by which it releases energy on addition of an electron.
anion from Cl atom in gaseous state. Chlorine atom need one electron to attain noble gas configuration. So, when an electron is added to the outer most shell of chlorine it attains stability of fully filled outermost shell by which it releases energy on addition of an electron.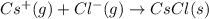
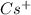 cation and
cation and 

