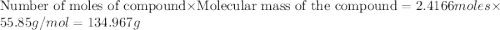Chemistry, 01.07.2019 08:30 edeliz2804
Al (s) + fe2o3 (s) --> al2o3 (s) + fe (s) (needs balancing) how many grams of fe can be produced when 65.2 g of al is reacted with an excess (unlimited) supply of fe2o3?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Where are each of the three particles located within the atom?
Answers: 1

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

You know the right answer?
Al (s) + fe2o3 (s) --> al2o3 (s) + fe (s) (needs balancing) how many grams of fe can be produced...
Questions


Mathematics, 16.10.2019 16:30


Mathematics, 16.10.2019 16:30


English, 16.10.2019 16:30



History, 16.10.2019 16:30


Medicine, 16.10.2019 16:30




English, 16.10.2019 16:30



Spanish, 16.10.2019 16:30


Mathematics, 16.10.2019 16:30

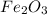 .
.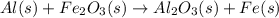
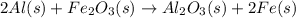
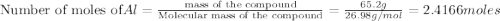
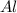 reacts with one mole of
reacts with one mole of 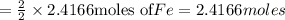
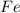 produced =
produced =