
Chemistry, 01.07.2019 11:00 ptonygonzalez701
Calculate the ph at the equivalence point for the titration of 0.230 m methylamine (ch3nh2) with 0.230 m hcl. the kb of methylamine is 5.0× 10–4.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
Calculate the ph at the equivalence point for the titration of 0.230 m methylamine (ch3nh2) with 0.2...
Questions



History, 25.02.2021 16:40

English, 25.02.2021 16:40

Computers and Technology, 25.02.2021 16:40



Mathematics, 25.02.2021 16:40

Mathematics, 25.02.2021 16:40





Mathematics, 25.02.2021 16:40

Physics, 25.02.2021 16:40



Health, 25.02.2021 16:40

Computers and Technology, 25.02.2021 16:40

![[OH^-]](/tpl/images/0038/4745/b2910.png) be x
be x![[CH3NH_2]](/tpl/images/0038/4745/f7563.png) , c = 0.230 M
, c = 0.230 M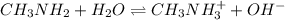
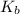 :
:![K_b=\frac{[CH_3NH_3^+][+OH^-]}{[CH_3NH_2]}=\frac{x\times x}{c-x}=\frac{x^2}{c-x}](/tpl/images/0038/4745/3e9a4.png)
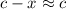 .
.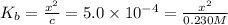
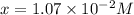
![[H^+]](/tpl/images/0038/4745/07acb.png) = 0.230 M
= 0.230 M![[OH^-]=[H^+]](/tpl/images/0038/4745/2bc09.png) will result in neutral solution, since
will result in neutral solution, since ![[OH^-]](/tpl/images/0038/4745/9eb9b.png)
![[H^+]_{\text{left in solution}}=[H^+]-[OH^-]=0.230-1.07\times 10^{-2}=0.2193 M](/tpl/images/0038/4745/6ec30.png)
![pH=-log{[H^+]_{\text{left in solution}}=-log(0.2193)=0.65](/tpl/images/0038/4745/0225f.png)


