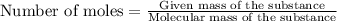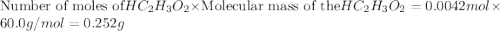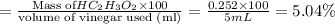The formula to determine the percent of hc2h3o2 (mass/volume) in vinegar is percent (m/v) = (grams of hc2h3o2/ volume of vinegar used (ml) ) x 100. if 5.0 ml of vinegar were used for the titration and 0.0042 moles of hc2h3o2 were required to reach the endpoint, calculate the percent of hc2h3o2 in vinegar. the molar mass of hc2h3o2 is 60.0 g / mol. question 10 options:

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2
You know the right answer?
The formula to determine the percent of hc2h3o2 (mass/volume) in vinegar is percent (m/v) = (grams o...
Questions


Mathematics, 15.04.2021 05:10



Mathematics, 15.04.2021 05:10

English, 15.04.2021 05:10


Arts, 15.04.2021 05:10

Mathematics, 15.04.2021 05:20

English, 15.04.2021 05:20

Mathematics, 15.04.2021 05:20



Social Studies, 15.04.2021 05:20

Geography, 15.04.2021 05:20

Mathematics, 15.04.2021 05:20


Mathematics, 15.04.2021 05:20

Mathematics, 15.04.2021 05:20

Mathematics, 15.04.2021 05:20

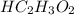 in vinegar 5.04%.
in vinegar 5.04%.