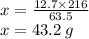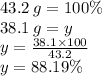Chemistry, 01.07.2019 13:30 rubincain203
In a particular reaction between copper metal and silver nitrate, 12.7 g cu produced 38.1 g ag. what is the percent yield of silver in this reaction? cu + 2agno3 → cu(no3)2 + 2ag a) 88.4% b) 176% c) 56.7% d) 77.3%

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

Chemistry, 22.06.2019 21:00
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1
You know the right answer?
In a particular reaction between copper metal and silver nitrate, 12.7 g cu produced 38.1 g ag. what...
Questions


Mathematics, 31.07.2019 05:30

Biology, 31.07.2019 05:30

Physics, 31.07.2019 05:30



Computers and Technology, 31.07.2019 05:30


Computers and Technology, 31.07.2019 05:30

History, 31.07.2019 05:30



Social Studies, 31.07.2019 05:30