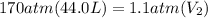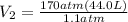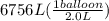Chemistry, 01.07.2019 17:30 2020davidhines
Acylinder containing 44.0 l of helium gas at a pressure of 170 atm is to be used to fill toy balloons to a pressure of 1.1 atm. each inflated balloon has a volume of 2.0 l. what is the maximum number of balloons that can be inflated? (remember that 44.0 l of helium at 1.1 atm pressure will remain in the 'exhausted' cylinder) round your answer to two significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1
You know the right answer?
Acylinder containing 44.0 l of helium gas at a pressure of 170 atm is to be used to fill toy balloon...
Questions



Mathematics, 10.11.2020 02:50


Mathematics, 10.11.2020 02:50



Mathematics, 10.11.2020 02:50

Computers and Technology, 10.11.2020 02:50


Mathematics, 10.11.2020 02:50

Mathematics, 10.11.2020 02:50


History, 10.11.2020 02:50

Mathematics, 10.11.2020 02:50


Mathematics, 10.11.2020 02:50


Health, 10.11.2020 02:50

Social Studies, 10.11.2020 02:50

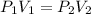
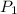 = 170 atm
= 170 atm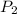 = 1.1 atm
= 1.1 atm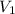 = 44.0 L
= 44.0 L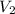 = ?
= ?