Chemistry, 01.07.2019 19:00 MahiraBashir
When two fluorine atoms bond together in f2, what type of covalent bond do they form? a double bond, because they overlap orbitals to share one pair of electrons. a double bond, because they overlap orbitals to share two pairs of electrons. a single bond, because they overlap orbitals to share one pair of electrons. a single bond, because they overlap orbitals to share two pairs of electrons. pls !

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
When two fluorine atoms bond together in f2, what type of covalent bond do they form? a double bond...
Questions

Mathematics, 29.10.2020 05:30

Social Studies, 29.10.2020 05:30

Chemistry, 29.10.2020 05:30

Mathematics, 29.10.2020 05:30

Mathematics, 29.10.2020 05:30







Mathematics, 29.10.2020 05:30

Chemistry, 29.10.2020 05:30

History, 29.10.2020 05:30

Mathematics, 29.10.2020 05:30




Mathematics, 29.10.2020 05:30

History, 29.10.2020 05:30

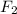 achieves a noble gas configuration.
achieves a noble gas configuration.