
Chemistry, 01.07.2019 19:00 gus2006santos
What volume of h2o is formed at stp when 6.0g of al is treated with excess naoh? naoh + al + h2o —-> naal(oh)4 + h2 (g)

Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:04
If this equation was completed which statement would it best support
Answers: 1

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3
You know the right answer?
What volume of h2o is formed at stp when 6.0g of al is treated with excess naoh? naoh + al + h2o —-...
Questions








Computers and Technology, 28.06.2019 02:30


Mathematics, 28.06.2019 02:30



History, 28.06.2019 02:30

History, 28.06.2019 02:30






Mathematics, 28.06.2019 02:30

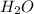 is 14.784 L.
is 14.784 L.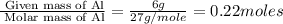
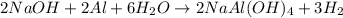
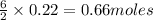 of
of 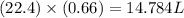 volume of
volume of 

