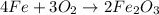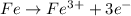Chemistry, 01.07.2019 19:30 minecraftsam2018
]in the chemical reaction shown below, which element was oxidized? 4fe + 3o2 yields 2fe2o3 fe, because it gained 3 electrons fe, because it lost 3 electrons o, because it gained 2 electrons o, because it lost 2 electrons

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1
You know the right answer?
]in the chemical reaction shown below, which element was oxidized? 4fe + 3o2 yields 2fe2o3 fe, beca...
Questions



Geography, 02.08.2019 10:40

Physics, 02.08.2019 10:40



History, 02.08.2019 10:40





Mathematics, 02.08.2019 10:40





Mathematics, 02.08.2019 10:40


Mathematics, 02.08.2019 10:40