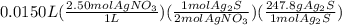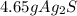Chemistry, 01.07.2019 21:30 jadenpmoore2008
Select the correct answer. if a silver nitrate solution is added to excess sodium sulfide, this reaction takes place: 2agno3(aq) + na2s(aq) → ag2s(s) + 2nano3(aq). suppose you use 0.0150 liter of a 2.50 m solution of silver nitrate. assuming the reaction goes to completion, how much silver sulfide is produced? use the periodic table. a. 1.49 g b. 4.65 g c. 9.30 g d. 18.6 g

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Diamond, graphite, and fullerenes share what property? a. they are all made of carbon (c) bonded to a metal. b. their shape. c. they are all made of carbon (c). d. they are all good conductors.
Answers: 1

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 23.06.2019 00:30
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3

Chemistry, 23.06.2019 07:00
Introduction of drugs into the gastrointestinal tract is a form of administration. a. enteral b. topical c. parenteral d. inhalation
Answers: 1
You know the right answer?
Select the correct answer. if a silver nitrate solution is added to excess sodium sulfide, this reac...
Questions



History, 15.12.2021 08:30



Mathematics, 15.12.2021 08:30

Mathematics, 15.12.2021 08:30



Mathematics, 15.12.2021 08:30



Mathematics, 15.12.2021 08:30


English, 15.12.2021 08:40

Mathematics, 15.12.2021 08:40

Mathematics, 15.12.2021 08:40


History, 15.12.2021 08:40

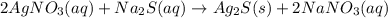
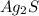 = 2(107.87)+32.06 = 247.8 g per mol
= 2(107.87)+32.06 = 247.8 g per mol