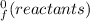Chemistry, 02.07.2019 02:30 hfroslie9840
What is the change in enthalpy for the following reaction? 2h2o2(aq)  2h2o(l) + o2(g) given: h2o: ∆h= -242 kjh2o2: ∆h= -609 kja.-286 kjb. -572 kjc. 572 kjd. 286 kj

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

You know the right answer?
What is the change in enthalpy for the following reaction? 2h2o2(aq)  2h2o(l)&nb...
Questions





History, 03.12.2019 16:31

Mathematics, 03.12.2019 16:31





Social Studies, 03.12.2019 16:31





History, 03.12.2019 16:31

Mathematics, 03.12.2019 16:31

Biology, 03.12.2019 16:31

Mathematics, 03.12.2019 16:31

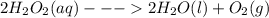
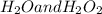 are:
are: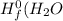 ) = -285.8kJ/mol[/tex]
) = -285.8kJ/mol[/tex]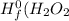 ) = -187.6 kJ/mol[/tex]
) = -187.6 kJ/mol[/tex]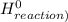 =∑ΔH
=∑ΔH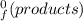 -∑ΔH
-∑ΔH