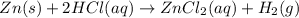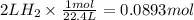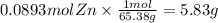How many grams of zinc metal are required to produce 2.00 liters of hydrogen gas at stp according to the chemical equation shown below? how many grams of zinc metal are required to produce 2.00 liters of hydrogen gas at stp according to the chemical equation shown below? 5.83 g 11.7 g 0.171 g 131 g?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3
You know the right answer?
How many grams of zinc metal are required to produce 2.00 liters of hydrogen gas at stp according to...
Questions

Chemistry, 05.10.2019 15:30



History, 05.10.2019 15:30


History, 05.10.2019 15:30

History, 05.10.2019 15:30

Mathematics, 05.10.2019 15:30

Mathematics, 05.10.2019 15:30

Mathematics, 05.10.2019 15:30

History, 05.10.2019 15:30

English, 05.10.2019 15:30

History, 05.10.2019 15:30

English, 05.10.2019 15:30

History, 05.10.2019 15:30

Mathematics, 05.10.2019 15:30


History, 05.10.2019 15:30

Mathematics, 05.10.2019 15:30

Biology, 05.10.2019 15:30