
Chemistry, 02.07.2019 04:00 qveenjordan6456
Which options correctly describe similarities between the elements boron (b), aluminum (al), and gallium (ga)? select all that apply. hint: review your periodic table. all three elements are part of the boron family. all three elements tend to form ionic compounds that cannot be dissolved in water. all three elements tend to form 3+ cations when reacting with metals. all three elements have five valence electrons.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1


Chemistry, 23.06.2019 18:30
The electron–pair geometry of a molecule is tetrahedral. what is its bond angle if there are no lone pairs of electrons?
Answers: 2
You know the right answer?
Which options correctly describe similarities between the elements boron (b), aluminum (al), and gal...
Questions

Computers and Technology, 23.06.2019 09:30

Mathematics, 23.06.2019 09:30


Health, 23.06.2019 09:30

Health, 23.06.2019 09:30


Mathematics, 23.06.2019 09:30

Mathematics, 23.06.2019 09:30

History, 23.06.2019 09:30




Mathematics, 23.06.2019 09:30



Mathematics, 23.06.2019 09:30

English, 23.06.2019 09:30



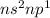 .Total number of valence electron in each element is 3.These elements show (+3) oxidation state. So, these elements tend to form (+3) cations when reacting with metals.
.Total number of valence electron in each element is 3.These elements show (+3) oxidation state. So, these elements tend to form (+3) cations when reacting with metals.



