
Which of the following statements is true about the strength of the intermolecular forces in ch4 and nh3? a: ch4 ≥ nh3 because ch4 is tetrahedral but nh3 is pyramidal. b: ch4 < nh3 because δ− on c in the ch bond is greater than δ− on n in the nh bond. c: ch4 < nh3 because the nh bond is more polar than the ch bond. d: ch4 ≥ nh3 because ch4 has h bonding but nh3 has dispersion forces.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
If equal masses of the listed metals were collected , which would have a greatest volume ? a. aluminum 2.70,b.zinc7.14,c.copper 8.92,d.lead 11.34
Answers: 2

Chemistry, 21.06.2019 21:00
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1
You know the right answer?
Which of the following statements is true about the strength of the intermolecular forces in ch4 and...
Questions




Business, 01.11.2019 01:31

Computers and Technology, 01.11.2019 01:31

Mathematics, 01.11.2019 01:31

Health, 01.11.2019 01:31





History, 01.11.2019 01:31

Mathematics, 01.11.2019 01:31





English, 01.11.2019 01:31

Mathematics, 01.11.2019 01:31

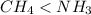 because the NH bond is more polar than CH bond.
because the NH bond is more polar than CH bond.

