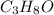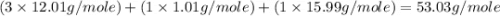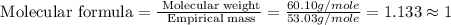Chemistry, 02.07.2019 06:00 wyndal1753
Determine the molecular formula of the compound. a compound is used as a gasoline additive. it has a molecular weight of 60.10 atomic mass units and an empirical formula of c3h8o. the molecular formula is

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
Determine the molecular formula of the compound. a compound is used as a gasoline additive. it has a...
Questions







Mathematics, 17.07.2019 13:00

Social Studies, 17.07.2019 13:00

Mathematics, 17.07.2019 13:00

Social Studies, 17.07.2019 13:00


Social Studies, 17.07.2019 13:00





Mathematics, 17.07.2019 13:00



English, 17.07.2019 13:00


 .
.