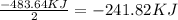Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2


Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1
You know the right answer?
Water forms according to the equation below: 2h2(g) + o2(g) jpg 2h2o(g) hrxn = -483.64 kj how much...
Questions


Computers and Technology, 29.07.2019 15:20


Biology, 29.07.2019 15:20

Mathematics, 29.07.2019 15:20


Biology, 29.07.2019 15:20


Mathematics, 29.07.2019 15:20



Mathematics, 29.07.2019 15:20


Biology, 29.07.2019 15:20




Geography, 29.07.2019 15:20

Health, 29.07.2019 15:20

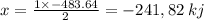

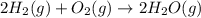
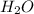 released amount of energy = -483.64 KJ
released amount of energy = -483.64 KJ