
Type the correct answer in the box. express the answer to three significant figures. given: ch4 + 2o2 → co2 + 2h2o, δh = -890 kj/mol how much energy is released when 59.7 grams of methane (ch4) reacts with oxygen? the combustion of 59.7 grams of methane releases 34.5 kilojoules of energy.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
Type the correct answer in the box. express the answer to three significant figures. given: ch4 + 2...
Questions


Mathematics, 02.11.2020 14:00


Mathematics, 02.11.2020 14:00

English, 02.11.2020 14:00


Advanced Placement (AP), 02.11.2020 14:00




Mathematics, 02.11.2020 14:00


Mathematics, 02.11.2020 14:00

Mathematics, 02.11.2020 14:00

History, 02.11.2020 14:00


Mathematics, 02.11.2020 14:00

Arts, 02.11.2020 14:00

Mathematics, 02.11.2020 14:00

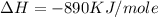
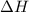 is in negative that means the energy is releasing.
is in negative that means the energy is releasing.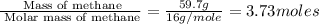
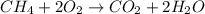
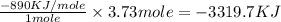 of energy
of energy

