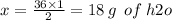Chemistry, 02.07.2019 17:00 lovelylid6969
According to the following equation: 2h2 + o2 → 2h2o how many grams of water will be produced from 1.0 mol hydrogen gas? 34.0 g h2o

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 06:00
How does a coronal mass ejection (cme) affect the solar wind? a cme adds more particles to the solar wind, intensifying it. a cme blocks the solar wind, causing it to fade. a cme does not affect the solar wind but it does affect auroras. a cme increases the amount of energy in the solar wind.
Answers: 2

Chemistry, 23.06.2019 06:30
Moving force of air flows from areas of high pressure to areas of low pressure true or false
Answers: 2

Chemistry, 23.06.2019 11:20
Sandy is building a small toy car. he wants to use a balloon to power the toy car. he fills a balloon with air and then attaches a straw to the balloon. he tapes the balloon-straw combination to the car and then releases the end of the balloon. the toy moves forward as the air from the balloon comes out the back of the straw. what can sandy do to make the toy car move faster? a) use less air in the balloon. b) blow up the balloon more. c) use a longer straw. d) use larger tires.
Answers: 2

Chemistry, 23.06.2019 21:00
Jenny takes a piece of paper and dipped it in a bowl of water the paper is now damp and mushy which of the following is our true about the change that occurs when the paper gets wet
Answers: 3
You know the right answer?
According to the following equation: 2h2 + o2 → 2h2o how many grams of water will be produced from...
Questions

Chemistry, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40


Health, 12.12.2020 16:40

English, 12.12.2020 16:40

English, 12.12.2020 16:40









Mathematics, 12.12.2020 16:40

Health, 12.12.2020 16:40



Mathematics, 12.12.2020 16:40