
Chemistry, 02.07.2019 17:00 2018jecr46871
You discover a chemical reaction that produces 1 moles of valuable propane for every 6 moles of carbon supplied. essentially, this is an exchange rate of 1 moles of propane for every 6 moles of carbon. if you have 1.8 moles of carbon, how many moles of propane can you produce using this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
What is the theoretical yield of carbon dioxide? a)0.993 gb)2.98 gc)3.65 gd)8.93 g
Answers: 1

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3
You know the right answer?
You discover a chemical reaction that produces 1 moles of valuable propane for every 6 moles of carb...
Questions

History, 27.06.2019 15:20


History, 27.06.2019 15:30


Mathematics, 27.06.2019 15:30

Mathematics, 27.06.2019 15:30



History, 27.06.2019 15:30



Mathematics, 27.06.2019 15:30

Mathematics, 27.06.2019 15:30


Mathematics, 27.06.2019 15:30

Mathematics, 27.06.2019 15:30




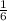 ×1.8 = 0.3 moles of the propane can be prepared.
×1.8 = 0.3 moles of the propane can be prepared.


