
Chemistry, 02.07.2019 18:30 anthonysutton82
In the reaction equation bro-3(aq) br-(aq) + bro-4(aq), how many oxidation states does the disproportionate substance have throughout the reaction? 0 1 2 3

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
You know the right answer?
In the reaction equation bro-3(aq) br-(aq) + bro-4(aq), how many oxidation states does the dispropor...
Questions




Physics, 02.02.2021 22:40


Mathematics, 02.02.2021 22:40



Biology, 02.02.2021 22:40





Mathematics, 02.02.2021 22:40




Mathematics, 02.02.2021 22:40

Health, 02.02.2021 22:40

Biology, 02.02.2021 22:40


 is (+5).
is (+5).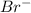 is (-1).
is (-1).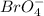 is (+7).
is (+7).

