
The molecule carbon disulfide cs2 is nonpolar and has only london dispersion forces between the molecules. carbon tetrachloride ccl4 is also nonploar but is made larger molecules that are more strongly affected by london dispersion forces. based on this information cs2 likely has a melting point and a vapor pressure when compared with ccl4

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Two nitro no2 groups are chemically bonded to a patch of surface. they can't move to another location on the surface, but they can rotate (see sketch at right). it turns out that the amount of rotational kinetic energy each no2 group can have is required to be a multiple of ε, where =ε×1.010−24 j. in other words, each no2 group could have ε of rotational kinetic energy, or 2ε, or 3ε, and so forth — but it cannot have just any old amount of rotational kinetic energy. suppose the total rotational kinetic energy in this system is initially known to be 32ε. then, some heat is removed from the system, and the total rotational kinetic energy falls to 18ε. calculate the change in entropy. round your answer to 3 significant digits, and be sure it has the correct unit symbol.
Answers: 2

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
The molecule carbon disulfide cs2 is nonpolar and has only london dispersion forces between the mole...
Questions


Mathematics, 21.04.2021 04:40


Mathematics, 21.04.2021 04:40

Mathematics, 21.04.2021 04:40

Mathematics, 21.04.2021 04:40



Physics, 21.04.2021 04:40



Mathematics, 21.04.2021 04:40


Mathematics, 21.04.2021 04:40




Mathematics, 21.04.2021 04:40

Mathematics, 21.04.2021 04:40

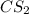 where as the vapor pressure of
where as the vapor pressure of ![CS_2[/tex[ is higher than [tex]CCl_4](/tpl/images/0043/9801/99b5e.png) .
.


