
Chemistry, 02.07.2019 22:00 ethanm2685
Question 30select the correct answer from each drop-down menu. a reaction proceeds with 2.72 moles of magnesium chlorate and 3.14 moles of sodium hydroxide. this is the equation of the reaction: mg(clo3)2 + 2naoh → mg(oh)2 + 2naclo3. determine the theoretical amount of each product that the reaction will produce. the reaction will produce of magnesium hydroxide and of sodium chlorate. (both have the same dropdown) a. 1.36 b. 1.57 c. 2.72 d. 3.14 e. 4.52 (all in moles)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2


Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1
You know the right answer?
Question 30select the correct answer from each drop-down menu. a reaction proceeds with 2.72 moles o...
Questions


Mathematics, 08.03.2021 01:00



Mathematics, 08.03.2021 01:00



Mathematics, 08.03.2021 01:00





Biology, 08.03.2021 01:00

Mathematics, 08.03.2021 01:00

Mathematics, 08.03.2021 01:00

Arts, 08.03.2021 01:00

Mathematics, 08.03.2021 01:00


Mathematics, 08.03.2021 01:00

Physics, 08.03.2021 01:00

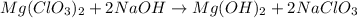
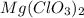 react with 2 mole of
react with 2 mole of 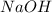
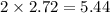 moles of
moles of 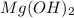 and
and 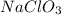 by using the moles of limiting reagent.
by using the moles of limiting reagent.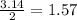 moles of
moles of 


