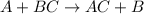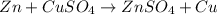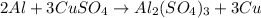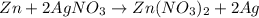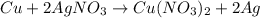30 pts! balancing equations. 2. for part 2: single-displacement reactions: for each of the four single-displacement reactions, describe what happened in each well. if a chemical reaction occurred, write a balanced equation for it. then using the a, b symbols, write a general equation for a single-displacement reaction. a+bc=ac+b here are the chemical formulas of the reactants for each reaction: • zinc – zn copper sulfate – cuso4 zn+cuso4-> cu+znso4 • aluminum – al copper sulfate – cuso4 no reaction • zinc – zn silver nitrate – ag(no3) • copper – cu silver nitrate – ag(no3)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Review the branily terms and services guides well u know what i never did so go have a nice ice cream sunday
Answers: 1

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2
You know the right answer?
30 pts! balancing equations. 2. for part 2: single-displacement reactions: for each of the four s...
Questions

History, 05.07.2019 07:30

Health, 05.07.2019 07:30


Social Studies, 05.07.2019 07:30

Mathematics, 05.07.2019 07:30



Mathematics, 05.07.2019 07:30

Mathematics, 05.07.2019 07:30

Mathematics, 05.07.2019 07:30


Biology, 05.07.2019 07:30

History, 05.07.2019 07:30

Geography, 05.07.2019 07:30

History, 05.07.2019 07:30

Mathematics, 05.07.2019 07:30


Biology, 05.07.2019 07:30