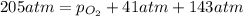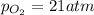Chemistry, 03.07.2019 01:00 FailingstudentXD
Ascuba diver’s air tank contains oxygen, helium, and nitrogen at a total pressure of 205 atmospheres. the partial pressure of nitrogen is 143 atmospheres, and the partial pressure of helium is 41 atmospheres. what is the partial pressure of oxygen in the tank? a. 21 atm b. 103 atm c. 307 atm d. 389 atm

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2

Chemistry, 23.06.2019 11:00
Which of the following reactions is endothermic? h2(g) + ½ o2(g) h2o(g), h = -57.82 kcal ½n2(g) + o2(g) + 8.1 kcal no2(g) ½ n2(g) + 3/2 h2(g) nh3(g) + 11.0 kcal c(diamond) + o2(g) co2, h = -94.50 kcal
Answers: 2
You know the right answer?
Ascuba diver’s air tank contains oxygen, helium, and nitrogen at a total pressure of 205 atmospheres...
Questions

Mathematics, 19.02.2021 19:50

Chemistry, 19.02.2021 19:50

Mathematics, 19.02.2021 19:50



Social Studies, 19.02.2021 19:50


Mathematics, 19.02.2021 19:50

History, 19.02.2021 19:50

Business, 19.02.2021 19:50



Mathematics, 19.02.2021 19:50


History, 19.02.2021 19:50





Mathematics, 19.02.2021 19:50

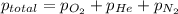
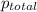 = 205 atm
= 205 atm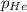 = 41 atm
= 41 atm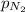 = 143 atm
= 143 atm