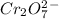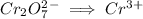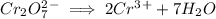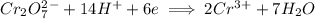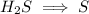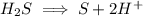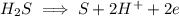Chemistry, 03.07.2019 01:00 babyboo6745
Write the half reactions for the following equation. identify which half reaction is oxidized and which is reduced. ag + no₃- --> ag+ + no write the half reactions for the following equation. identify which half reaction is oxidized and which is reduced. zn + no₃- --> zn²+ + no₂ write the half reactions for the following equation. identify which half reaction is oxidized and which is reduced. cr₂o₇ ²- (aq) + h₂s(g) --> cr³+(aq) + s(s)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2
You know the right answer?
Write the half reactions for the following equation. identify which half reaction is oxidized and w...
Questions

Computers and Technology, 14.12.2019 03:31



















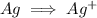
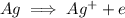
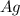 is being oxidized. Oxidation is loss of electrons.
is being oxidized. Oxidation is loss of electrons. 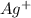
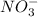

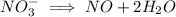
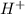 ions on left hand side to balance hyrdogen.
ions on left hand side to balance hyrdogen.
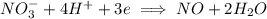
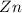
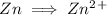
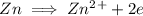
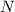 is being reduced since it is moving from a +5 oxidation state to a +2 oxidation state by gaining electrons.
is being reduced since it is moving from a +5 oxidation state to a +2 oxidation state by gaining electrons.