
Chemistry, 03.07.2019 01:30 idontknow11223344
Using iupac nomenclature, give the name of bh3 and alh3 and elaborate why that name is correct. a) boron trihydride & aluminum trihydride - both molecules combine two non-metals and form covalent bonds. b) boron hydride & aluminum hydride - both compounds combine a main group metal and a non-metal to form ionic bonds. c) boron trihydride - boron and hydrogen are both non-metals and form covalent bonds. aluminum hydride - aluminum is a main group metal and hydrogen is a non-metal and they form ionic bonds. d) boron hydride - boron is a main group metal and hydrogen is a non-metal and they form ionic bonds. aluminum trihydride - aluminum and hydrogen are both non-metals and form covalent bonds.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1
You know the right answer?
Using iupac nomenclature, give the name of bh3 and alh3 and elaborate why that name is correct. a)...
Questions




Chemistry, 21.04.2021 16:50


Mathematics, 21.04.2021 16:50


Mathematics, 21.04.2021 16:50

Mathematics, 21.04.2021 16:50

Mathematics, 21.04.2021 16:50

Mathematics, 21.04.2021 16:50



Mathematics, 21.04.2021 16:50






Mathematics, 21.04.2021 16:50

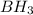 is a covalent compound because sharing of electrons takes place between hydrogen and boron. Both the elements are non-metals and hence, will form covalent bond.
is a covalent compound because sharing of electrons takes place between hydrogen and boron. Both the elements are non-metals and hence, will form covalent bond.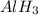 is an ionic compound because aluminium element is a metal and hydrogen element is a non-metal. The bond formed between a metal and a non-metal is always ionic in nature.
is an ionic compound because aluminium element is a metal and hydrogen element is a non-metal. The bond formed between a metal and a non-metal is always ionic in nature.

