
Chemistry, 03.07.2019 03:30 makeda2010
He atomic number of fluorine is 9. how many electrons does an ion of fluorine have if it is represented by the symbol shown below? 19f1- a. 8 electrons b. 9 electrons c. 10 electrons d. 20 electrons

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
He atomic number of fluorine is 9. how many electrons does an ion of fluorine have if it is represen...
Questions


Physics, 03.07.2019 23:50

Biology, 03.07.2019 23:50


Mathematics, 03.07.2019 23:50

Mathematics, 03.07.2019 23:50

Mathematics, 03.07.2019 23:50

Social Studies, 03.07.2019 23:50

Mathematics, 03.07.2019 23:50


Mathematics, 03.07.2019 23:50




Mathematics, 03.07.2019 23:50

Mathematics, 03.07.2019 23:50

History, 03.07.2019 23:50

Social Studies, 03.07.2019 23:50

Mathematics, 03.07.2019 23:50

Chemistry, 03.07.2019 23:50

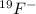 . It represents a uninegative anion with a mass number of 19.
. It represents a uninegative anion with a mass number of 19.

