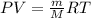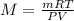Chemistry, 03.07.2019 04:00 1230bering
Ascientist has been working in a lab with several containers, each containing a different noble gas. he is worried that he may have mistakenly swapped the labels on two containers. one container is filled with non-toxic helium gas. the second container is filled with the toxic, highly carcinogenic noble gas, radon. the scientist knows the volume, temperature, and pressure of both of the gas containers. he also knows the mass of an empty container. he does not want to open the containers to figure out what the two gases are, for fear of releasing toxic radon into the lab. which of the following would most likely the scientist identify the gases? a. re-measure the temperature of the containers. b. measure the mass of the gases in each container. c. measure the speed of the molecules in each container. d. measure the charges of the electrons in each of the containers. a scientist has been working in a lab with several containers, each containing a different noble gas. he is worried that he may have mistakenly swapped the labels on two containers. one container is filled with non-toxic helium gas. the second container is filled with the toxic, highly carcinogenic noble gas, radon. the scientist knows the volume, temperature, and pressure of both of the gas containers. he also knows the mass of an empty container. he does not want to open the containers to figure out what the two gases are, for fear of releasing toxic radon into the lab. which of the following would most likely the scientist identify the gases? a. re-measure the temperature of the containers. b. measure the mass of the gases in each container. c. measure the speed of the molecules in each container. d. measure the charges of the electrons in each of the containers.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1

Chemistry, 23.06.2019 07:30
In the diagram here that represents the reaction, which reactant, a or b, is the limiting reagent?
Answers: 1
You know the right answer?
Ascientist has been working in a lab with several containers, each containing a different noble gas....
Questions

Mathematics, 23.01.2020 07:31

Mathematics, 23.01.2020 07:31

History, 23.01.2020 07:31



Mathematics, 23.01.2020 07:31

Mathematics, 23.01.2020 07:31

Business, 23.01.2020 07:31



Mathematics, 23.01.2020 07:31



Computers and Technology, 23.01.2020 07:31

Spanish, 23.01.2020 07:31



English, 23.01.2020 07:31

Mathematics, 23.01.2020 07:31