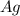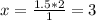Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which function is performed by earths atmosphere? a. ultraviolet rays are prevented from reaching the ozone layer. b. earths temperature is raised and moderated by trapping in heat c. charged particles from the sun are prevented from reaching earth. d. magnetic charges from space are prevented from reaching earths surface.
Answers: 2

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
You know the right answer?
If 1.5 moles of copper metal react with 4.0 moles of silver nitrate, how many moles of silver metal...
Questions

History, 02.09.2020 02:01

Mathematics, 02.09.2020 02:01

Geography, 02.09.2020 02:01

Biology, 02.09.2020 02:01


English, 02.09.2020 02:01


Mathematics, 02.09.2020 02:01

Biology, 02.09.2020 02:01



History, 02.09.2020 02:01




Mathematics, 02.09.2020 02:01

Mathematics, 02.09.2020 02:01

Mathematics, 02.09.2020 02:01

History, 02.09.2020 02:01

Social Studies, 02.09.2020 02:01

 will be left.
will be left.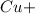 2
2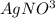 →
→ 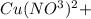 2
2