Chemistry, 03.07.2019 10:30 kaliahgrey
Use the balanced equation to work the following problem: cacl2 + 2agno3 → 2 agcl + ca(no3)2 how many grams of agcl (molar mass=143 g/mol) is formed when 1.00 gram of agno3 (molar mass=170 g/mol) reacts? 1.00 g 0.00588 g 1.19 g 0.841 g

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1
You know the right answer?
Use the balanced equation to work the following problem: cacl2 + 2agno3 → 2 agcl + ca(no3)2 how man...
Questions


Mathematics, 05.10.2019 00:00




Mathematics, 05.10.2019 00:00

English, 05.10.2019 00:00




History, 05.10.2019 00:00




History, 05.10.2019 00:00



History, 05.10.2019 00:00

Biology, 05.10.2019 00:00

English, 05.10.2019 00:00

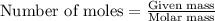
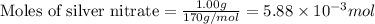
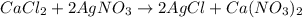
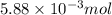 of silver nitrate will produce =
of silver nitrate will produce = 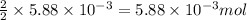 of AgCl
of AgCl