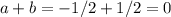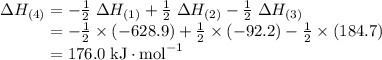Chemistry, 03.07.2019 12:30 adrielalvarez267
Given the following data: n2(g) + 4h2(g) + cl2(g) → 2nh4cl(s) δh = -628.9 kj n2(g) + 3h2(g) → 2nh3(g) δh = -92.2 kj 2hcl(g) → h2(g) + cl2(g) δh = 184.7 kj find the δh of the following reaction: nh4cl(s) → nh3(g) + hcl(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1


Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2
You know the right answer?
Given the following data: n2(g) + 4h2(g) + cl2(g) → 2nh4cl(s) δh = -628.9 kj n2(g) + 3h2(g) → 2nh3...
Questions

English, 13.02.2021 21:40

Mathematics, 13.02.2021 21:40

History, 13.02.2021 21:40

English, 13.02.2021 21:40

English, 13.02.2021 21:40

Mathematics, 13.02.2021 21:40


Mathematics, 13.02.2021 21:40

Business, 13.02.2021 21:40

Mathematics, 13.02.2021 21:40

English, 13.02.2021 21:40


Mathematics, 13.02.2021 21:40

English, 13.02.2021 21:40

Mathematics, 13.02.2021 21:40

Chemistry, 13.02.2021 21:40


Mathematics, 13.02.2021 21:40

Mathematics, 13.02.2021 21:40

Mathematics, 13.02.2021 21:40

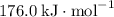
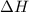 can be determined with the Hess's Law. The key is to find the appropriate coefficient for each of the given equations.
can be determined with the Hess's Law. The key is to find the appropriate coefficient for each of the given equations.  ,
, 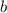 , and
, and  be letters such that
be letters such that 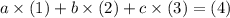 . This relationship shall hold for all chemicals involved.
. This relationship shall hold for all chemicals involved. 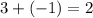 shall resemble the number of
shall resemble the number of 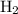 left on the product side when the second equation is directly added to the third. Similarly
left on the product side when the second equation is directly added to the third. Similarly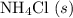 :
: 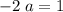
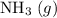 :
: 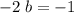
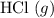 :
: 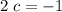
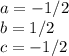 and
and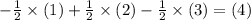
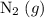 for instance. Nitrogen isn't present in the net equation. The sum of its coefficient shall, therefore, be zero.
for instance. Nitrogen isn't present in the net equation. The sum of its coefficient shall, therefore, be zero.