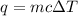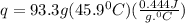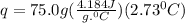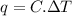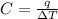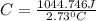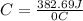Chemistry, 03.07.2019 13:00 xmanavongrove55
Acalorimeter contained 75.0 g of water at 16.95 c. a 93.3-g sample of iron at 65.58 c was placed in it, giving a final temperature of 19.68 c for the system. calculate the heat capacity of the calorimeter. specific heats are 4.184 j/g c for h2o and 0.444 j/g c for fe.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 23.06.2019 10:30
Ireally need ! calcium metal reacts with a potassium chloride solution to form calcium chloride and potassium ions. balance this reaction. (s) + (aq) → cacl2(s) + +(aq) a) 1, 2, 1, 2 b) 1, 2, 1, 1 c) 1, 1, 1, 1 d) 2, 1, 2, 1
Answers: 1

Chemistry, 23.06.2019 12:30
If you reacted 450 g of trimethylgallium with 300 g of arsine, what mass of gaas could you make?
Answers: 1

Chemistry, 23.06.2019 15:00
The atoms in a have a definite volume, but move quickly enough to overcome the forces of attraction between them. a. solid b.liquid c.gas
Answers: 2
You know the right answer?
Acalorimeter contained 75.0 g of water at 16.95 c. a 93.3-g sample of iron at 65.58 c was placed in...
Questions




History, 13.02.2020 20:23

English, 13.02.2020 20:23













Computers and Technology, 13.02.2020 20:23


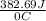 .
. for iron metal = 65.58 - 19.68 = 45.9 degree C
for iron metal = 65.58 - 19.68 = 45.9 degree C