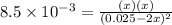Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3
You know the right answer?
For the equilibrium 2 ibr (g) i2 (g) + br2 (g) kp=8.5 ×10-3 at 150 oc. if 0.025 atm of ibr is place...
Questions


Mathematics, 15.12.2020 19:10

History, 15.12.2020 19:10

Mathematics, 15.12.2020 19:10

Chemistry, 15.12.2020 19:10





Biology, 15.12.2020 19:10


History, 15.12.2020 19:10

Mathematics, 15.12.2020 19:10

Mathematics, 15.12.2020 19:10

Physics, 15.12.2020 19:10





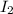 and
and 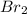 is 0.0211 atm, 0.00195 atm and 0.00195 atm respectively.
is 0.0211 atm, 0.00195 atm and 0.00195 atm respectively.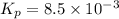
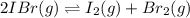
![K_p=\frac{[P_I_2][P_{Br}_2]}{[P_{IBr}]^2}](/tpl/images/0046/9546/983fc.png)