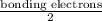Chemistry, 03.07.2019 16:30 EssenceBlocker144
Consider the lewis structure for the polyatomic oxyanion shown here, where x is an element from the third period (na−ar). by changing the overall charge, n, from 1- to 2- to 3- we get three different polyatomic ions. element x is surrounded by 4 oxygen atoms bonded to it with full octets. a)for each of these ions identify the central atom, x. arrange your answers in order increasing n. b)for each of these ions determine the formal charge of the central atom, x. arrange your answers in order increasing n. i really have no idea how to solve this. any would be greatly appreciated!

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3


Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
Consider the lewis structure for the polyatomic oxyanion shown here, where x is an element from the...
Questions










Chemistry, 07.04.2020 21:59

Physics, 07.04.2020 22:00




English, 07.04.2020 22:00