Two solutions namely, 500 ml of 0.50 m hcl and 500 ml of 0.50 m naoh at the same temperature of 21.6 are mixed in a constant-pressure calorimeter. the heat capacity of the calorimeter was 450 j/c. given that the specific heat of the solution is 4.184 j/gc, the density of the solution is 1.0 g/ml, and that the heat of neutralization for the process h+oh=h2o is -56.2 kj, what is the final temperature of the mixed solution

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2
You know the right answer?
Two solutions namely, 500 ml of 0.50 m hcl and 500 ml of 0.50 m naoh at the same temperature of 21.6...
Questions











Computers and Technology, 25.01.2020 00:31



Computers and Technology, 25.01.2020 00:31






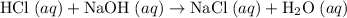
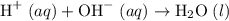
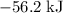 . Thus the combination of every mole of hydrogen ions and hydroxide ions in solution would produce
. Thus the combination of every mole of hydrogen ions and hydroxide ions in solution would produce 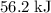 or
or 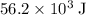 of energy.
of energy.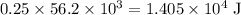 of energy.
of energy. 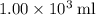 of the 1.0 gram per milliliter solution. Accordingly, it would have a mass of
of the 1.0 gram per milliliter solution. Accordingly, it would have a mass of 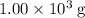 .
. 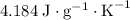 . The solution thus have a heat capacity of
. The solution thus have a heat capacity of 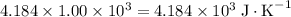 . Note that one degree Kelvins K is equivalent to one degree celsius ℃ in temperature change measurements.
. Note that one degree Kelvins K is equivalent to one degree celsius ℃ in temperature change measurements.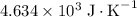 , meaning that its temperature would rise by 1 degree celsius on the absorption of 4.634 × 10³ joules of energy.
, meaning that its temperature would rise by 1 degree celsius on the absorption of 4.634 × 10³ joules of energy.  are available from the reaction. Thus, the temperature of the system shall have risen by 3.03 degrees celsius to 24.6 degrees celsius by the end of the reaction.
are available from the reaction. Thus, the temperature of the system shall have risen by 3.03 degrees celsius to 24.6 degrees celsius by the end of the reaction.