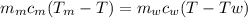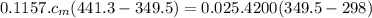Ahot lump of 115.7 g of an unknown substance initially at 168.3°c is placed in 25.0 ml of water initially at 25.0°c and allowed to reach thermal equilibrium. the final temperature of the system is 76.5°c. what is the identity of the unknown substance? assume no heat is lost to the surroundings

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2



You know the right answer?
Ahot lump of 115.7 g of an unknown substance initially at 168.3°c is placed in 25.0 ml of water init...
Questions

History, 14.11.2019 19:31



History, 14.11.2019 19:31


Mathematics, 14.11.2019 19:31

Mathematics, 14.11.2019 19:31


Spanish, 14.11.2019 19:31

History, 14.11.2019 19:31

Health, 14.11.2019 19:31

Biology, 14.11.2019 19:31








 .
.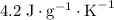 and a density of
and a density of 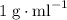 . 25.0 milliliters of water thus has a mass of 25.0 grams.
. 25.0 milliliters of water thus has a mass of 25.0 grams. 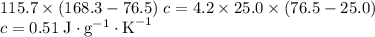
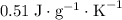 . This substance is thus probably steel.
. This substance is thus probably steel.