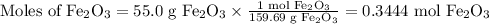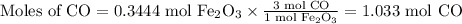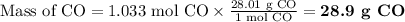Chemistry, 04.07.2019 05:30 thelonewolf5020
What mass of co is needed to react completely with55.0 g of fe2o3(s)+co(g) yield fe(s)+co2(g)?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

You know the right answer?
What mass of co is needed to react completely with55.0 g of fe2o3(s)+co(g) yield fe(s)+co2(g)?...
Questions



Mathematics, 12.05.2021 01:30

Mathematics, 12.05.2021 01:30






Chemistry, 12.05.2021 01:30

Mathematics, 12.05.2021 01:30


Chemistry, 12.05.2021 01:30


History, 12.05.2021 01:30



Mathematics, 12.05.2021 01:30


Business, 12.05.2021 01:30

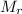 : 159.69 28.01
: 159.69 28.01