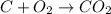Chemistry, 04.07.2019 09:30 smithmalyk4
View the diagram below describe what happened in the diagram. the molecules on both sides of the chemical reaction are the same, because they are both mixtures. bonds were broken on the reactants and new bonds were formed on the products. the molecules expanded and cooled. bonds were broken on the reactants and no new bonds were formed on the products.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

You know the right answer?
View the diagram below describe what happened in the diagram. the molecules on both sides of the che...
Questions

Mathematics, 21.03.2021 22:50

Mathematics, 21.03.2021 22:50


Mathematics, 21.03.2021 22:50


Spanish, 21.03.2021 22:50



SAT, 21.03.2021 22:50

Social Studies, 21.03.2021 22:50


Advanced Placement (AP), 21.03.2021 22:50

Computers and Technology, 21.03.2021 22:50

Spanish, 21.03.2021 22:50


English, 21.03.2021 22:50


Mathematics, 21.03.2021 22:50

Chemistry, 21.03.2021 22:50

Chemistry, 21.03.2021 22:50