
Chemistry, 04.07.2019 10:00 kenken2583
A50.00 g sample of an unknown metal is heated to 45.00°c. it is then placed in a coffee-cup calorimeter filled with water. the calorimeter and the water have a combined mass of 250.0 g and an overall specific heat of 1.035 cal/g•°c. the initial temperature of the calorimeter is 10.00°c. the system reaches a final temperature of 11.08°c when the metal is added. any heat lost by the metal is

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1
You know the right answer?
A50.00 g sample of an unknown metal is heated to 45.00°c. it is then placed in a coffee-cup calorime...
Questions




Mathematics, 14.11.2020 05:40

Mathematics, 14.11.2020 05:40

Biology, 14.11.2020 05:40

Social Studies, 14.11.2020 05:40



Mathematics, 14.11.2020 05:40

Mathematics, 14.11.2020 05:40

Mathematics, 14.11.2020 05:40


History, 14.11.2020 05:40


Mathematics, 14.11.2020 05:40


Mathematics, 14.11.2020 05:40


Biology, 14.11.2020 05:40

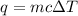 .
. is change in temperature.
is change in temperature.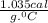 .
. 

