
Chemistry, 04.07.2019 10:30 wrightdarius78
Which reaction is a decomposition reaction? a. 2kclo3 → 2kcl + 3o2 b. 4na + o2 → 2na2o c. zns + 3o2 → 2zno + 2so2 d. 2nabr + caf2 → 2naf + cabr2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
Which reaction is a decomposition reaction? a. 2kclo3 → 2kcl + 3o2 b. 4na + o2 → 2na2o c. zns + 3o2...
Questions

History, 04.08.2019 16:30

Chemistry, 04.08.2019 16:30

Chemistry, 04.08.2019 16:30




Chemistry, 04.08.2019 16:30


Mathematics, 04.08.2019 16:30




Mathematics, 04.08.2019 16:30




Mathematics, 04.08.2019 16:30


Mathematics, 04.08.2019 16:30

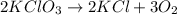
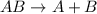
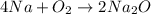 this reaction is a combination reaction in which the two or more molecules combine to form a larger single molecule as a product.
this reaction is a combination reaction in which the two or more molecules combine to form a larger single molecule as a product.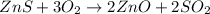 this is a double displacement reaction in which the cation and the anion of the two reactant exchange their places to give two another compounds as a product.
this is a double displacement reaction in which the cation and the anion of the two reactant exchange their places to give two another compounds as a product.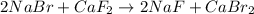 this is a double displacement reaction in which the cation and the anion of the two reactant exchange their places to give two another compounds as a product.
this is a double displacement reaction in which the cation and the anion of the two reactant exchange their places to give two another compounds as a product.

