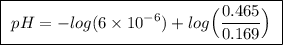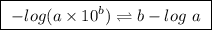Chemistry, 04.07.2019 11:00 rntaran2002
Abuffer solution is made by dissolving 0.45 moles of a weak acid (ha) and 0.33 moles of koh into 710 ml of solution. what is the ph of this buffer? ka = 6x10-6 for ha.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

You know the right answer?
Abuffer solution is made by dissolving 0.45 moles of a weak acid (ha) and 0.33 moles of koh into 710...
Questions

Arts, 26.12.2019 06:31





Mathematics, 26.12.2019 06:31

Social Studies, 26.12.2019 06:31



History, 26.12.2019 06:31


Biology, 26.12.2019 06:31


Biology, 26.12.2019 06:31

English, 26.12.2019 06:31



Physics, 26.12.2019 06:31


Physics, 26.12.2019 06:31

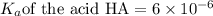
![[concentration]=\frac{\text{number of moles}}{\text{volume in litres}}](/tpl/images/0049/9814/01f13.png)
![[HA]=\frac{0.45}{0.710} mol/L,[KOH]=\frac{0.33}{0.710} mol/L](/tpl/images/0049/9814/a3a14.png)
![pH=pK_a+log\frac{[base]}{[acid]}](/tpl/images/0049/9814/9facd.png) ...(1)
...(1)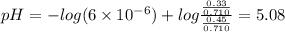
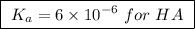
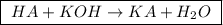
![\boxed{ \ pH = pKa + log_{10} \frac{[A-]}{[HA]} \ }](/tpl/images/0049/9814/90294.png)
![\boxed{ \ [HA] = \frac{0.12 \ moles}{0.710 \ L} = 0.169 \ M \ }](/tpl/images/0049/9814/115c3.png)
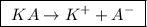 we observe that there is one A⁻ ion or the valence is 1.
we observe that there is one A⁻ ion or the valence is 1. ![\boxed{ \ [A^-] = [KA] \times valence \ }](/tpl/images/0049/9814/39c11.png) , so
, so![\boxed{ \ [A^-] = \frac{0.33 \ moles}{0.710 \ L} \times 1 = 0.465 \ M \ }](/tpl/images/0049/9814/6cb5e.png)